Technical Evolution and Identification of Resin-Filled Turquoise

ABSTRACT
Zhushan County is the most abundant source of commercial turquoise in China’s Hubei Province, where mass production has been ongoing for several decades. Chinese turquoise production and consumption have created a significant industry and promoted local economic prosperity and development. Treatment techniques for turquoise have been updated and changed rapidly, sometimes even monthly. Traditionally, rough materials are treated with resin to reduce their high porosity to improve processability, appearance, and stability. In this research, the characteristics of resin-filled turquoise were investigated by ultraviolet fluorescence, spectrofluorometry, and Fourier-transform infrared spectrometry. The differences in three-dimensional fluorescence patterns between untreated and resin-filled turquoise are reported for the first time. The three-dimensional fluorescence patterns along with the ultraviolet reactions and infrared spectra allow for the identification of this filling. Unlike untreated turquoise, the resin-filled turquoise samples had a strong and main emission peak centered at approximately 444 nm and a secondary emission shoulder at approximately 460 nm under approximately 372 nm wavelength excitation in this research. This study sheds light on resin filling processes by observing the essential features of 342 turquoise specimens after treatment. Resin filling can decrease the porosity of turquoise to improve its color and its value, and this study provides identification guidelines for gemologists and useful information on the resin-filling process.
INTRODUCTION
Turquoise, a hydrated copper aluminum phosphate mineral (CuAl6(PO4)4(OH)8•4H2O), has been appreciated as a valuable gem material worldwide for thousands of years. It has a wide range of colors, such as blue, blue-green, green, yellowish green, and greenish yellow. Among these colors, blue turquoise is the most popular and sought after in the commercial market (figure 1).
In the early 1980s, many residents of Shiyan City in China’s Hubei Province owned turquoise, though few were conscious of its value. The locally produced turquoise was a great bargain at about US$100–$200/kg. Moreover, small fragments were even used there as outdoor decorative materials in the 1980s (figure 2). In 2009, the local government suddenly realized the significance of its turquoise resources and started to manage mining. Since then, prices have soared because of limited turquoise resources and regulation of mining.

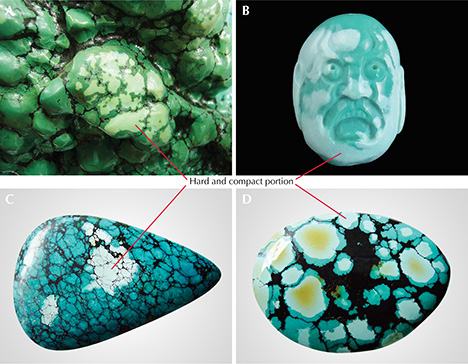
Generally, the portion of rough turquoise suitable for processing into fashioned gemstones is much less than 5% of the mined total. Most natural turquoise contains fissures, pores, matrix, or other defects (figure 3, left and center). Much of the porous turquoise (figure 3, right) is termed “pale” or “chalky.” This material’s low hardness makes it unsuitable for processing, and it is often accompanied by low color saturation and numerous micrometer- to nanometer-sized pores. With daily wear, the color and luster of turquoise may change gradually from prolonged absorption of sweat and skin oil. Nevertheless, many Chinese turquoise enthusiasts and collectors are passionate about its natural appearance and variations.

As a porous cryptocrystalline aggregate, rough turquoise has been subjected to filling treatment to make it easier to process and improve its appearance and stability (Koivula et al., 1992; Choudhary, 2010; McClure et al., 2010). Filling treatment has been performed on a variety of low-quality gemstones with pores, fractures, or fissures. Examples include opal (Gambhir, 2001; Filin and Puzynin, 2009; Hu et al., 2013), tourmaline (Deng et al., 2009), emerald (Kammerling et al., 1991; Johnson et al., 1999; Johnson, 2007), and ruby (“GAAJ Lab Alert,” 2004; McClure et al., 2006).

In China, conventional pore fillers used for turquoise are wax and resin. The appearance and durability of “pale” or “chalky” turquoise can be greatly improved by pore filling to decrease its porosity (figure 4). Using this technique, porous material can be fully optimized to increase its value. Occasionally, however, treated turquoise products are unfavorable due to the material’s uneven hardness. The resin agent permeates and infiltrates porous areas much more easily than the compact portion. Consequently, the porous portions develop a more saturated color than the compact portion after resin treatment. As a result, the resin-treated turquoise displays a mottled appearance with obvious and artificial boundaries after polishing (figure 5). Resin filling of material sold in China should be noted in a turquoise identification report according to the Chinese national testing standard.
Turquoise can also be impregnated with a mixture of resin, hardener, and colorant. However, such impregnation and dyeing treatment is unacceptable in the Chinese commercial trade. Additionally, the “Zachery” treatment developed in the United States can be applied to improve the color and polish of turquoise, which was first reported by G&G in 1999 (Fritsch et al., 1999). However, the specific equipment and agents used in the Zachery technique remain proprietary, and so far it has not been widely used in Hubei Province. Although Chinese manufacturers were initially unsure how this treatment works, they have tried to master the technology and succeeded in developing an electrochemical method (similar to the Zachery technique) to optimize not only blue turquoise, but also yellow and green materials. The thicker treated layer and saturated color produced by this electrochemical method are a significant improvement (Lin et al., 2019; Shen et al., 2018). Moreover, oiling is also widely applied to turquoise in the Chinese market today (Koivula et al., 1992; Hu et al., 2019). For a glossary of turquoise enhancement terminology, see box A.
|
BOX A: Terminology of Turquoise Treatment Techniques |
|
Waxing: Turquoise (generally fashioned turquoise) is immersed in wax to coat its surface to enhance durability and appearance and prevent discoloration over time. This treatment is accepted in the trade. Oiling: Turquoise is treated for several days or weeks by various types of oil (e.g., mineral or vegetable oil), which acts as a concealer and filler to hide superficial flaws (such as white spots) and decrease porosity for better color, appearance, and stability. Dyeing: Turquoise is colored by various dyes or colored substances. Resin filling: Under ambient conditions, resin and hardener are introduced into the pores of the turquoise to obtain a more stable material for processability and durability. With filling of the pores, the color of the turquoise improves because there is less light scattering. Resin impregnation: Turquoise is impregnated with a combination of resin and hardener. The resulting altered color is dull and saturated. This process is different from resin filling. Both vacuum and pressure conditions are applied in the impregnation process but not in resin filling. Resin impregnation and dyeing: Colorants are added and mixed with the resin and hardener. This treatment process is also performed under vacuum as well as pressure conditions, similar to resin impregnation. Porcelain enhancement: A popular treatment technique that decreases the porosity of turquoise and improves the compactness, hardness, and luster without the use of organic polymers. Typically, this treatment involves filling turquoise with aluminum dihydrogen phosphate or sodium silicate. If turquoise is treated with phosphate substances, colorant could have been added if deemed necessary. If treated with sodium silicate, however, there is no addition of colorant. Zachery treatment: Turquoise is treated with a potassium-containing compound for better stability and appearance. Its name refers to entrepreneurial electrical engineer James E. Zachery, who invented this process in the late 1980s (Fritsch et al., 1999). Electrochemical method: Rough or fashioned turquoise is treated with electrolytes (usually containing potassium) based on electrochemical fundamentals (similar to the Zachery technique). The treated turquoise can achieve more saturated color not only on the surface but throughouts the entire sample (Lin et al., 2019; Shen et al., 2018). Yellow/black-fading: Turquoise is treated with chemical agents to get rid of black or yellow stain-like colors. |
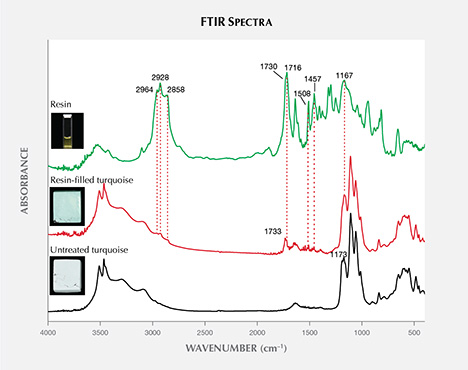
Researchers have devoted great effort to the identification of treated turquoise, as well as various kinds of fillers such as resin, polymer, oil, plastic, and dyed substances (Lind et al., 1983; Chen et al., 2006; Moe et al., 2007; Chen et al., 2010a; Choudhary, 2010; McClure et al., 2010; Han et al., 2015; Bernardino et al., 2016; Zuo et al., 2017; Xu and Di, 2018; Hu et al., 2019; Liu et al., 2019; Xu and Yang, 2019). The treated examples mentioned above can be easily identified by different testing techniques, such as Raman and IR spectroscopy (Henn and Milisenda, 2005; Chen et al., 2006; Moe et al., 2007; Zuo et al., 2017), UV-Vis spectrophotometry (Han et al., 2015), energy-dispersive X-ray fluorescence (EDXRF; Fritsch et al., 1999), and pyrolysis–gas chromatography–mass spectrometry (Py-GC-MS; Schwarzinger and Schwarzinger, 2017). An intense band at ~1730 cm–1 is detected in the IR spectra of most organic-treated turquoise (i.e., polymer, plastic, or resin), which is assigned to the C=O stretching vibration. Elevated potassium is detectable exclusively in Zachery-treated turquoise (Fritsch et al., 1999).
The usual analytical techniques, such as IR spectroscopy, are sensitive to organic compounds. Counterfeiters have cunningly applied new inorganic substances to achieve superior color, luster, texture, and porcelain-like quality to pass inspection and cater to consumer demand for high-quality turquoise. More recently, many new approaches to turquoise treatment have emerged in the market using various inorganic fillers, such as aluminum dihydrogen phosphate (Zhou and Yuan, 2008; Chen et al., 2010b) and sodium silicate (also called “glass resin”) (Deng et al., 2019). Few characteristic absorptions assignable to these fillers can be found in IR spectra and X-ray diffraction patterns, although scanning electron microscopy (SEM) is capable of revealing the anomalous structural characteristics of turquoise treated with aluminum phosphate adhesive (Zhou and Yuan, 2008; Chen et al., 2010b). Furthermore, some special chemical agents are used to get rid of black or yellow stain-like colors. Industry sources call this technique “yellow/black-fading” treatment and claim these stained turquoise materials can display the desired blue color after processing. Further investigation is necessary for this new treatment. Because treatment techniques have evolved so rapidly in China, their identification remains a great challenge. Some fillers are difficult to detect, and turquoise treated with inorganic substances exhibits nondescript reactions to routine and nondestructive examinations.
The pores of untreated and electrochemical-treated turquoise have been quantitatively analyzed in previous reports with the gas adsorption method and the X-ray micro-CT 3D imaging technique (Li et al., 2016; Lu et al., 2020). However, few reports have directly revealed the differences in characteristics and porosity between untreated and resin-filled turquoise (Liu et al., 2019; Xu and Yang, 2019).
This research systematically reports on resin filling techniques and the characteristics used to identify them. It also provides new insight into turquoise pores, as well as variations in the distinct features of untreated and resin-filled turquoise, namely their color and porosity. Additionally, this research aims to demonstrate the influence of pore filling on turquoise durability and appearance. The results provide valuable guidelines for detection and treatment.
Materials and Methods
Samples. Turquoise specimens were prepared by different methods and labeled as group A, B, or C. Samples in group A and those in group C from a rough sample were provided by local dealers. Group B samples were purchased from a local dealer.
Group A. A set of 342 pieces of turquoise with various colors, sizes, and shapes were treated with resin and hardener in three different factories located in Zhushan County. Prior to filling, the samples were opaque, with a dull to glassy luster. Their colors ranged from pale to deep blue, green-blue, green, or yellow.
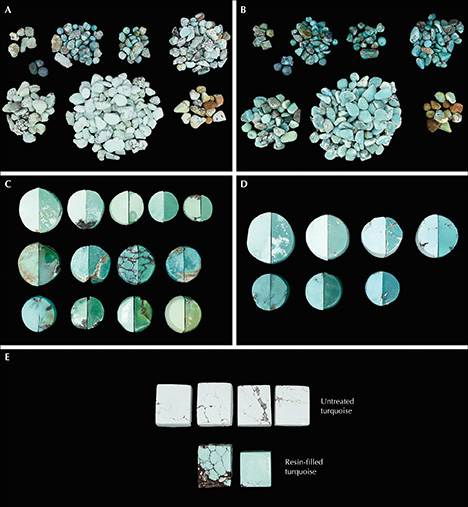
Group B. Twenty circular specimens of turquoise with various specific gravities (2.19–2.84) were selected for pore observation. Each sample was cut in half; one half was treated with resin and hardener to enhance the color, while the other was left untreated for comparison.

Group C. A porous and chalky rough sample (figure 6, left) from Zhushan County was cut into a series of block specimens, but we selected two block samples for testing in this study. These specimens were subsequently divided into two groups: (1) untreated and polished and (2) treated with mixed agents of resin and hardener (hereafter referred to as “resin”) (figure 6, right) for 2–3 days and then polished.
Characterization Techniques. Specific gravity was determined hydrostatically at the gem testing center of the China University of Geosciences (Wuhan) in Zhushan County. Images of all the samples were captured in a light box (D55 light source) under identical conditions to compare changes in color. Each group was characterized by different techniques (table 1).
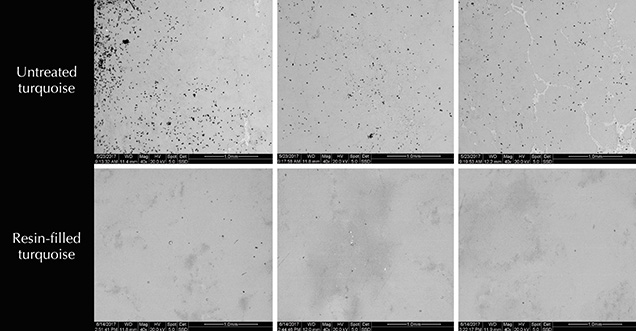
Environmental scanning electron microscopy (ESEM) (FEI Quanta 200) was performed on the 20 untreated halves (group B), as well as two block specimens (group C). All samples were coated with carbon and tested in a vacuum at a voltage of 20 kV.
Ultraviolet fluorescence was observed under short-wave (SW, 254 nm) and long-wave (LW, 365 nm) UV radiation. 3D fluorescent patterns of untreated, resin-filled block specimens (group C), and resin were collected by a Jasco FP-8500 spectrofluorometer with a xenon lamp light source. The photomultiplier tube (PMT) voltage was adjusted for sample testing (520 V for untreated and resin-filled turquoise, 550 V for resin). The emission range (270–750 nm) was measured with the excitation ranging from 250 to 600 nm at a response time of 0.5 seconds and scan speed of 2000 nm/min. The Ex and Em bandwidths were set to 5.0 and 2.5 nm, respectively.
Fourier transform infrared (FTIR) spectroscopy was recorded in the range of 400–4000 cm–1 with 64 scans at a resolution of 4.0 cm–1 using a Bruker VERTEX 80 FTIR spectrometer. The spectra of the untreated and resin-filled block specimens from group C, as well as the mixed agent of resin and hardener, were obtained using the KBr pellet method in transmission mode.
Resin Filling Process. Resin filling aims to enhance the texture or compactness of natural rough turquoise, which crumbles easily and is difficult to process due to its porous structure. The treatment can improve processability, durability, and stability, while also preventing the absorption of sweat, skin oil, and cosmetics. The procedures for our experiments were as follows (figure 7, left):
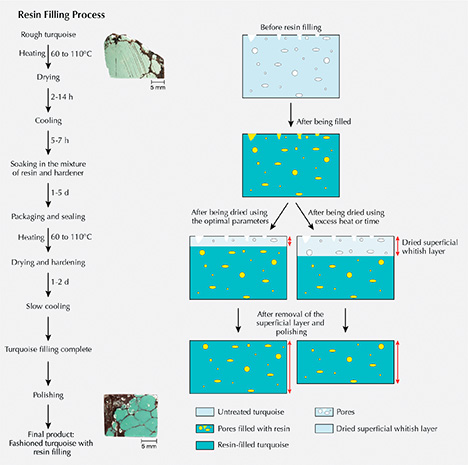
- Preheating and First Drying. Turquoise needs to be dried by preheating in an oven because it is somewhat hygroscopic and contains water. The initial temperature was set at about 60–80°C and maintained for 2–8 hours depending on sample dimensions. Subsequently, the temperature was increased at a rate of 5–20°C every 2–8 hours. The optimal temperature was raised to ~110°C until all of the absorbed water was evaporated.
- Cooling. The samples were slowly cooled down to room temperature over 5–7 hours. Turquoise should be protected from sharp dips in temperature, which can be destructive.
- Soaking. The dried turquoise was soaked in a mixture of resin and hardener for 1–5 days at room temperature without pressure until it was adequately filled, and it was then packaged and sealed.
- Second Drying for Hardening. Afterward, the samples were placed in an oven for secondary drying and hardening at a preliminary temperature of about 50–90°C for about 3.5 hours. The temperature was then increased by 5–10°C every five hours until peaking at 110°C. After the hardening procedure was completed, the oven was allowed to cool by 20°C every 2 hours until reaching room temperature.
- Finally, the filled turquoise samples were polished to remove the remaining fillers and superficial whitish dried layer to achieve the improved appearance.
The temperatures and durations given here are approximate because the processing factories requested confidentiality. Optimal parameters mattered significantly for resin filling. Temperature and dwell time should be carefully adjusted according to the size and porosity of the turquoise, as excessive heat and durations damage its inner structure. In addition, a whitish dried layer is produced on the surface of the turquoise after the secondary drying process. If the secondary drying stage (for hardening) was too long, material would be wasted as a thick whitish dried layer would form and need to be removed after resin filling (figure 7, right).
RESULTS
After resin filling, the treated samples were opaque, with a superficial whitish layer; see figure 7 (right) and figure 8 (center). The fashioned treated turquoise displayed obviously enhanced color and luster after polishing (figure 8).

Changes in Turquoise Color After Resin Filling. Most of the samples showed distinct improvement in color after resin filling (figure 9). The most pronounced color changes were observed in chalky and whitish samples with very high porosity, which turned noticeably more saturated in color after the filling. Those samples that had deep color and compact structure were only slightly modified, with barely perceptible color changes discerned since agents would not easily permeate the more compact turquoise.
Changes in Turquoise Porosity After Resin Filling. The dark and irregular areas in the ESEM images of turquoise represent surface pores (figure 10). The pores were unevenly distributed and sometimes crowded together, ranging from 20 to 200 μm in diameter with various shapes (circle, triangle, rhombus, or wormhole). Very few pores were observed in the ESEM images of the resin-filled sample. The untreated turquoise had higher porosity (approximately 1.9% surface porosity) than the resin-filled one (approximately 0.7%). The reduction of porosity confirmed that the resin effectively lowered the porosity and improved the appearance of samples.
Identification of the Treated Turquoise. UV Fluorescence. Untreated turquoise displayed an inert or very weak fluorescence reaction to both short- and long-wave UV. Resin-filled turquoise samples showed no fluorescence under short-wave UV radiation but strong blue fluorescence under long-wave UV (figure 11). The resin was inert to short-wave UV radiation but showed strong milky blue fluorescence under long-wave UV (again, see figure 11).

3D Fluorescence Patterns. There were distinct differences between the 3D fluorescence of untreated and resin-filled samples. Untreated turquoise fluoresced very weakly to the full excitation wavelength range (figure 12). The resin-filled turquoise, however, had a strong main emission peak centered at 444 nm under excitation at 372 nm, as well as a secondary emission shoulder at 460 nm.
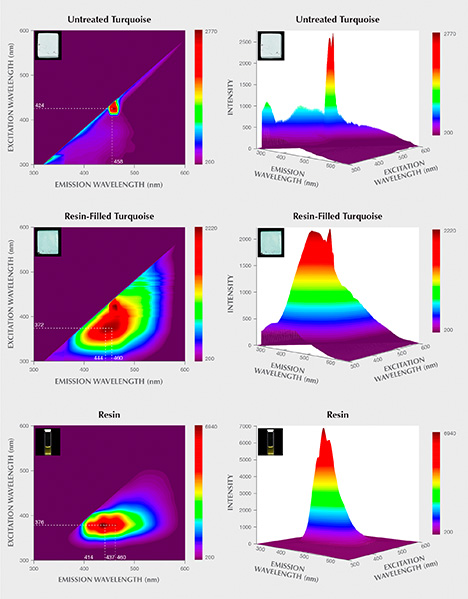
The 3D fluorescence pattern of the resin was also investigated in this study. The pattern exhibited three strong emission peaks centered at 414, 437, and 460 nm when excited by 376 nm light. These three strong emission centers occurring in the resin’s 3D fluorescence pattern matched well with those of the resin-filled turquoise. Accordingly, it was assumed that the presence of these strong emission peaks offered proof of resin filling. It should be noted that the varied types and dosages of organic substances used for resin treatment in each factory might alter the 3D fluorescence patterns of the resin-filled turquoise. The 3D fluorescence patterns of various resin-treated turquoise need to be further investigated.
Infrared Spectroscopy. The untreated turquoise showed important bands in two ranges, from 3000–3600 cm–1 and 1800–400 cm–1, which are typical variations of OH, H2O, and PO4 according to previous research (Reddy et al., 2006; Chen and Qi, 2007; Chen et al., 2012; Cejka et al., 2015). The spectra of the resin-filled turquoise were characterized by the different combinations of turquoise and resin used in the treatment process. As expected, the filled turquoise samples exhibited additional diagnostic bands of resin at approximately 1733 cm–1 (figure 13), attributable to C=O vibration (Lind et al., 1983; Pavese et al., 2005; Chen et al., 2006; Moe et al., 2007). Other weak bands appeared at 2964, 2928, 2858, 1716, 1508, 1457, and 1167 cm–1 in the spectrum of resin-filled turquoise. The resin is responsible for these bands. It displayed prominent bands at 2964, 2928, 2858, 1730, 1716, 1637, 1509, 1455, 1405, 1377, 1319, 1296, 1251, 1167, 1044, 940, 815, and 656 cm–1, as well as other weak bands.
There were notable differences in the fluorescence reactions and FTIR spectra of the untreated and treated turquoise, which offered diagnostic evidence for identifying resin-filled turquoise.
CONCLUSIONS
Resin filling can effectively improve the appearance of porous rough turquoise. Observations of pore distribution and quantity in both untreated and resin-filled turquoise reveal that most pores can be well filled by resin, resulting in a deeper color without the use of additional colorant. Untreated turquoise fluoresced very weakly to the full excitation wavelengths of light. But the resin-filled turquoise in this research had strong fluorescence under long-wave UV. Under 372 nm excitation, a strong main emission peak centered at 444 nm and a secondary emission shoulder at 460 nm occurred in its 3D fluorescence patterns. The filled turquoise had an intense band at 1733 cm–1, and other weak bands in IR spectra as well. Fluorescence reactions and the occurrence of additional FTIR bands related to organic resin provide the evidence required to identify resin-filled turquoise.
Pore filling is an economically worthwhile approach to optimize porous rough turquoise, making it suitable for commercial jewelry or ornaments with high added value. The effectiveness of pore-filling treatment in improving the processability, appearance, and durability of this precious gemstone should be recognized, and it needs to be fully disclosed to the consumer.



